Chemical Bonding
Every element and piece of matter on our planet is made up of tiny structures called atoms.
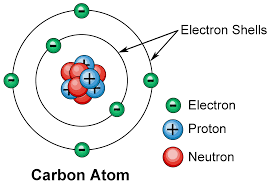
These small units of matter consist of several different parts, which include the nucleus, protons, neutrons, and electrons.
Each type of matter has a different amount of protons, which is what makes each element unique.
The number of protons is what gives an element its atomic number, which you can see on the periodic table. The number of electrons is always the same as the number of protons.
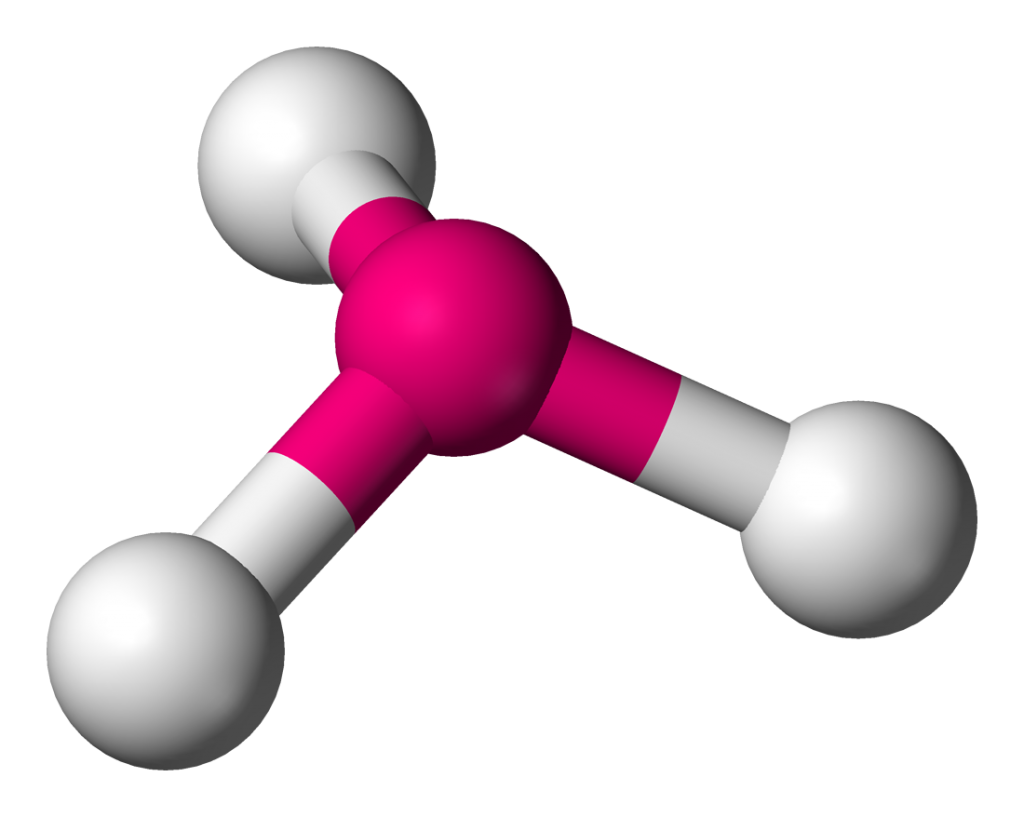
Atoms are so small, however, that it always takes more than one of them to form a larger piece of an element or matter.
The way that atoms form larger structures is through a process called chemical bonding, which allows the atoms to stick together.
Electron Shells
Electrons, unlike protons and neutrons, are not found in the center of an atom. Instead, they are orbiting the nucleus, much like our planet orbits the sun.
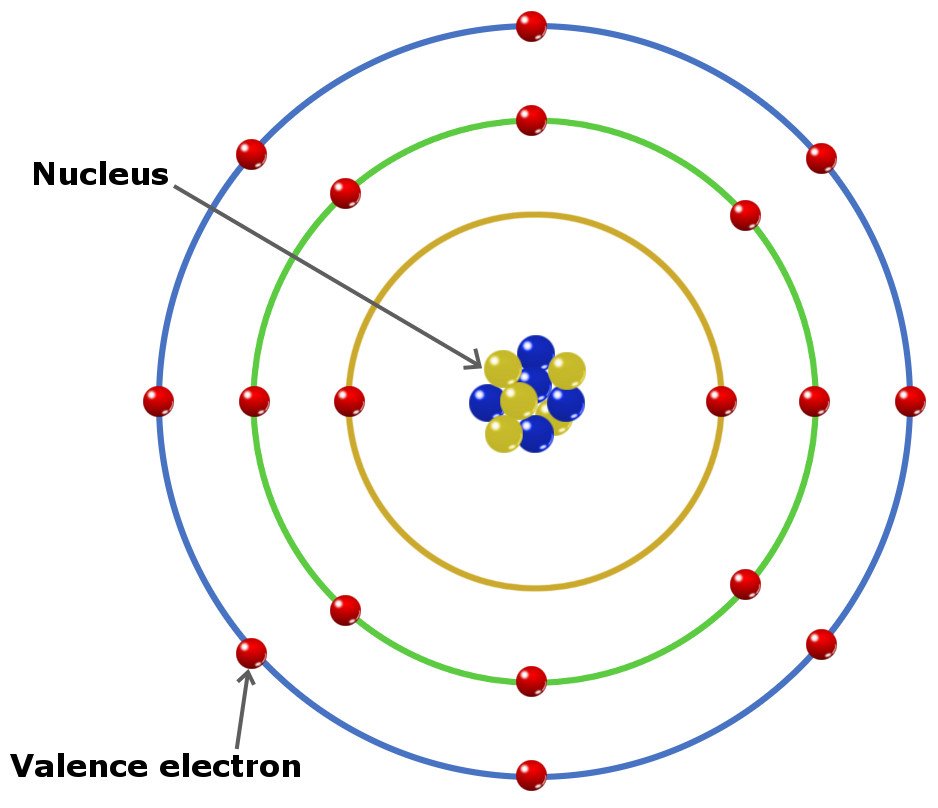
They do this in different layers, called shells.
Each shell can only contain a certain number of electrons, with the closest shell having the smallest amount and the further shells containing the most.
The Outer Shell
Atoms like to have a full outer shell, though this doesn’t happen too often in natural elements.
The only elements to have a full outer shell are the noble gases.
This need to have a full outer shell helps in the bonding process since atoms will share electrons with each other so that every atom in a piece of matter has a full outer shell.
Valence Electrons
The electrons that can participate in a chemical bond in the outer shell of an atom are known as valence electrons. Like you read above, atoms always want to have a full outer shell.
If an atom’s outer shell is relatively empty, then it will want to give up those electrons to another atom so that they both have a full outer shell.
On the other hand, if an outer shell is almost full, it will look to gain some electrons to ensure that it is filled up.
Two Types of Bonds
When it comes to chemical bonding, two different types can occur.
These two types of bonds are known as covalent bonding and ionic bonding.
Each of these bonding types utilizes electrons differently to ensure that all of the atoms have a full outer shell.
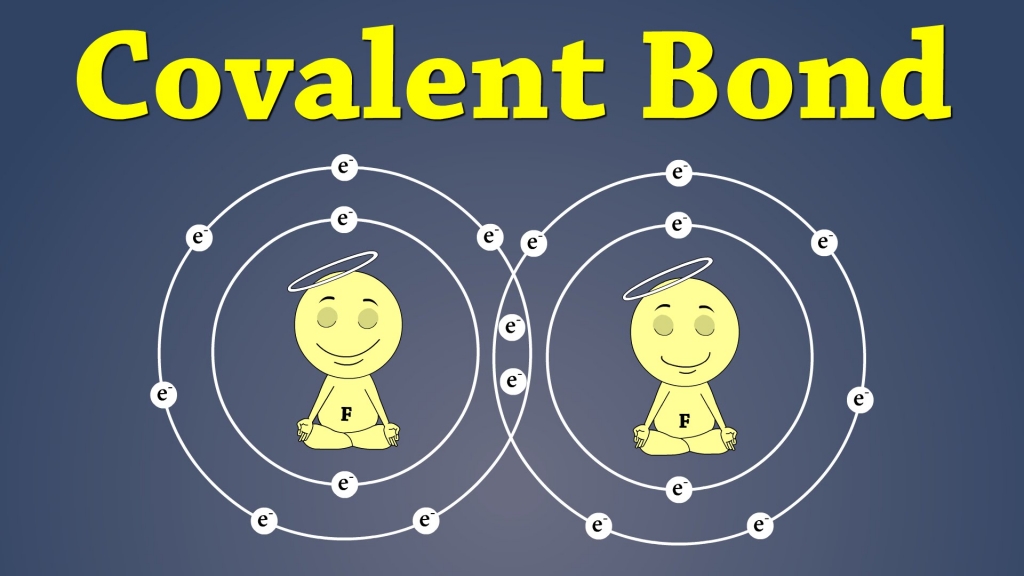
Covalent Bonding
In this type of bonding process, neither of the atoms will lose or gain an electron.
Instead, they share their electrons to make sure that they all have full outer shells.
Electrons are always shared in pairs between atoms.
Ionic Bonding
An ionic bond is formed when atoms from one element give up their electrons to another element so that they both can have a full outer shell.
Cool Facts About Chemical Bonding
The atoms within molecules are held together because of the attraction between the protons in the nucleus and the shared electrons in the shells.
Noble gases don’t usually react with other elements since they have a naturally full outer shell.