Melting and Boiling Facts
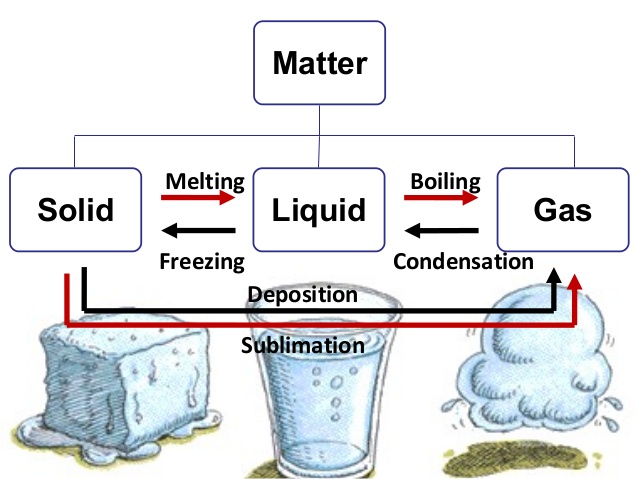
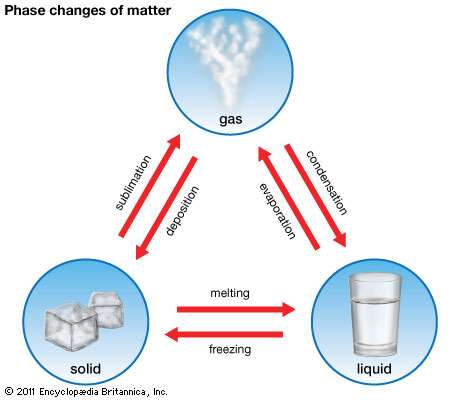
Every type of matter can be found in one state or another. These states, which can also be referred to as phases, are solids, liquids, and gases.
But how does matter change from one state to another? All you need to do is apply some heat and pressure.
The amount of heat/pressure that needs to be applied to change a state of matter depends on what kind of matter you are working with.
Some elements or matter don’t need a great deal of heat to change phases, while others require enormously high temperatures in order to move into the next state.
Standard State
If you ever hear a scientist mention the phrase “standard state”, you should know this refers to the state an element or piece of matter is most likely to be in at normal temperatures.
Pretty much every element’s standard state is that of a solid. This is true for substances such as lead or tin.
There are only two elements with a liquid standard state, which are mercury and bromine.
Hydrogen, nitrogen, and oxygen are all gases in their standard state.
Melting
As you have read before, the only way to change the state of matter for any given substance is through heat. If you are talking about changing a solid to a liquid, then this is a process called melting.
Every element and piece of matter in the world has a melting point, though some have a much higher melting temperature than others.
When an element is heated, its molecules gain more energy and begin to more about more freely.
This changes the element from a solid to a liquid.
Boiling
If an element or piece of matter is going to be changed from a liquid to a gas, even more heat will need to be applied. This will allow the substance to reach what is known as its boiling point.
Once the substance has been heated enough to reach its boiling point, the molecules will begin to move about even more freely, turning the substance from a liquid to a gas.
Every type of liquid has a boiling point, though some are much higher than others.
Evaporation
Another way that a liquid can be changed to a gas is through a process known as evaporation.
This type of change doesn’t need high temperatures, since it will not change all of the liquid at the same time.
Instead, if a liquid is left under a small heat source, the molecules at the surface will heat up, allowing them to become a gas, but leaving the rest of the liquid intact.
Related: States of Matter Facts
Freezing and Condensation
This process can also work in reverse. If heat is removed from a substance, it can be changed from a liquid to a solid or a gas to a liquid.
When a gas is changed to a liquid, the process is called condensation.
If a liquid is turned back into a solid, it is called freezing.
The temperatures for this to occur depend on what type of matter or element is being changed.