Molecules
Atoms are not usually found in isolation. They are mostly found joined together to form different kinds of substances. Whenever two atoms join together, they form a molecule.
Everything around you is made up of different kinds of molecules.
This also includes your body and the bodies of everyone that you have ever met!
Related: Try our molecule quiz here!
Did you know we are all made up of trillions upon trillions of different kinds of molecules?
Compounds
If atoms from different elements join together, they form a special type of molecule called a compound.
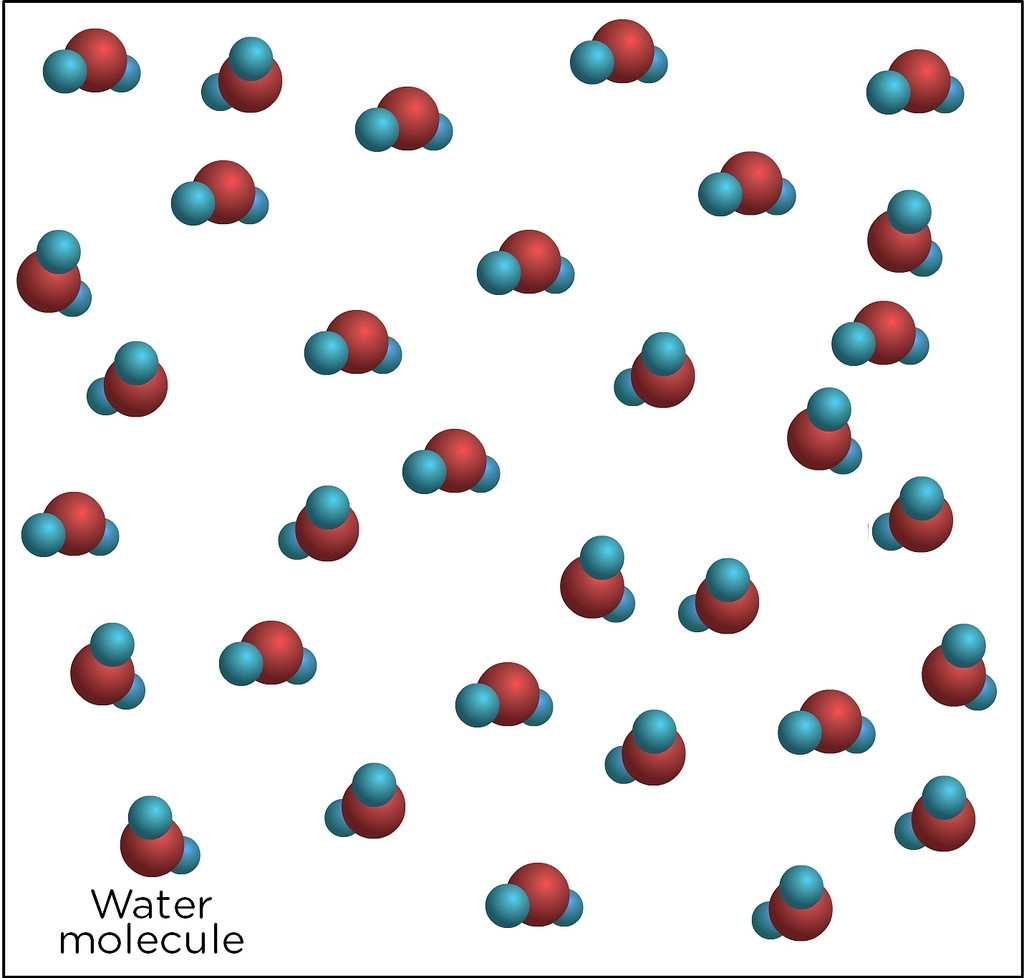
One of the most common compounds found in the world is also one of the most simple: water.
Water is made of compound molecules that are a combination of two hydrogen atoms and one oxygen atom.
This is why water is sometimes referred to as H2O.
No matter how much water there is, each molecule will always be made up of two hydrogen atoms and one oxygen.
Molecular Formula
Whenever you make a cake or cookies, you need to follow the right ingredients or formula. It is the exact same thing when different substances are created.
There are only about 100 different kinds of atoms in the world, but there are millions of different substances.
This is because they each have a unique formula (recipe) that is used to create them. Depending on the substance that is being created, there will be a different ratio of elements that are combined in order to create the substance.
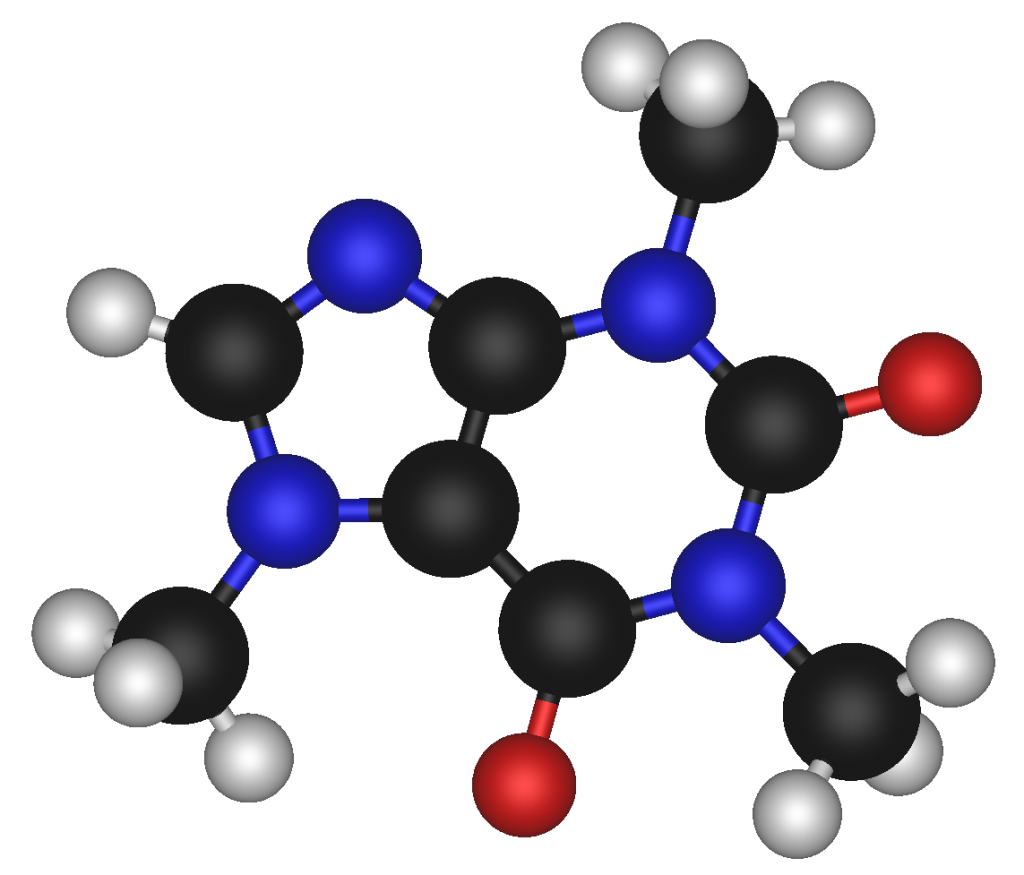
Depending on what is being examined, some of the different formulas can be either simple, such as water (H2O), or extremely complex, such as caffeine (C8H10N4O2).
The only way that any specific substance can be created is by having the proper kinds of atoms in the correct numbers.
Bonds
The molecules within compounds are held together by things called chemical bonds.
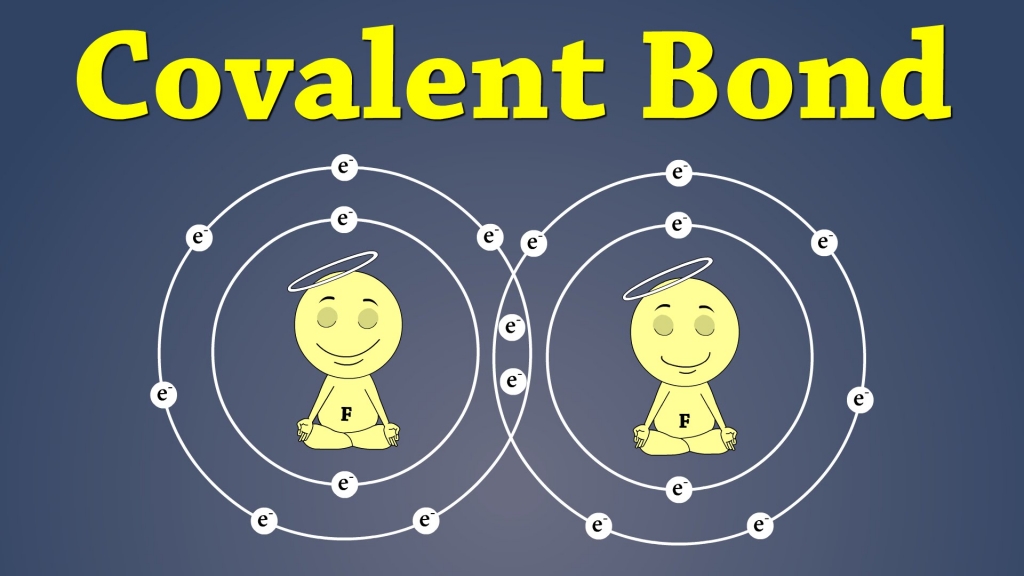
There are two different kinds of bonds that hold molecules together. These two different kinds of bonds are known as covalent and ionic bonds.
Compounds can have one or both kinds of bonds in their makeup.
These bonds are created when electrons fuse together. Basically, electrons orbit atoms in something like a shell, and these shells “like” to be full of electrons.
If the shells aren’t full, then they will bond with another atom to get the correct number of electrons.
Covalent Bonds – Covalent bonds form when electrons are shared between atoms.
Ionic Bonds – Ionic bonds form when an electron leaves one atom to join another.
This is done so that each atom has the correct number of electrons so that each one has a “full” shell.
Molecule Quiz
Now, give our quiz on molecules a go to test what you know!