Scandium Facts – What is scandium?
Scandium is a transition metal found in the first row of the third column on the periodic table of elements.
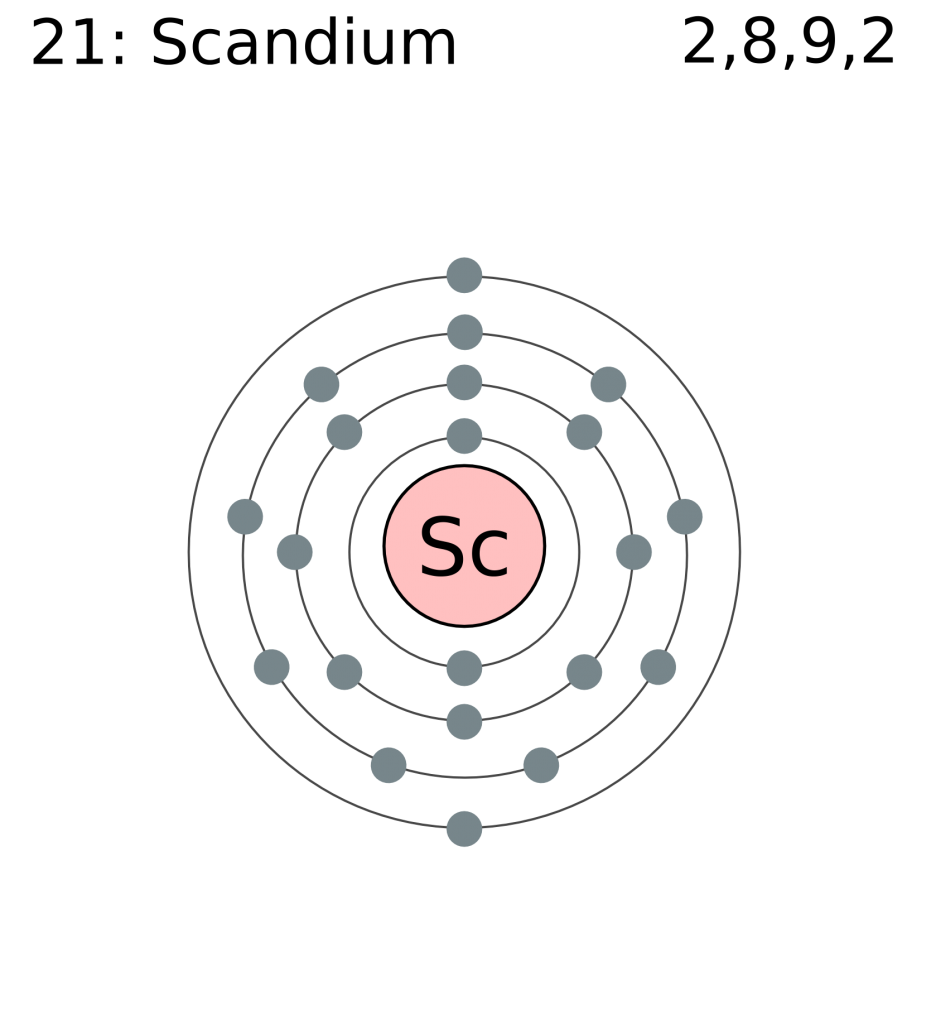
Its chemical symbol is Sc, and its atomic number is 21.
This is because it has 21 protons and 21 electrons.
The atomic weight of scandium is 44.96, and it is solid at room temperature.
Scandium’s melting point is a very high 1541 degrees Celsius, and its boiling point is also very high at 2836 degrees Celsius.
This means that scandium can retain its shape, structure, and overall form even at very high temperatures.
Lars Fredrik Nilson is credited with the discovery of scandium in the year 1879.
Characteristics and Properties
The standard appearance of scandium is a solid metal that has a silvery and white color to it. It is known to be quite soft in nature, and is very lightweight, much like aluminum.
Scandium does have very minor reactions, and when exposed to air it will become tarnished from its silvery color to a lighter pink or yellowish color.
Similarly, scandium has only minor reactions with acids. However, when exposed specifically to pure oxygen, scandium has no reaction.
In general it is protected and resistant to corrosion from exposure to other elements, and rust and decomposition.
It is generally very much like aluminum in appearance and function, except that it retains its shape and general form in very high temperatures.
This means that sometimes aluminum needs to be replaced with scandium for certain functions.
Where is scandium found on Earth?
Scandium is not a very common element to come across on Earth, and it is known to be only the 50th most abundant element found in the world.
Generally, scandium can only be found in small amounts at a time, mixed with other minerals in the Earth’s crust.
Scandium is not commonly mined or produced on its own, and is much more commonly found as a side result of mining and purifying fluorine, tantalum, and even uranium.
How scandium is used today
Scandium is a useful element when combined with others into a new metal alloy.
However, the cost of finding and using scandium outweighs its ability to be useful, and thus it is generally not a commonly used element.
Aluminum and titanium are much more common in use than scandium, due to their similar properties, strength, and cost effectiveness.
Combined, alloys of scandium and aluminum can make very strong and highly durable products.
Examples of products that come from aluminum and scandium alloys include aircraft parts, firearms, baseball bats, and golf clubs.
Another example of a scandium-based product is extremely bright light, commonly used in movie production and to light arenas for major events.
It is very hard to isolate and purify scandium. It is much more common to both find and use it in some form of compound, ore, or alloy.
The first pound of purified scandium wasn’t produced until the year 1960.
Although scandium isn’t extremely common on Earth, in outer space and amongst the stars, scandium is extremely common, even ranking as the 26th most abundant element in space.
Fun Scandium Quiz!
- What year was the first pound of scandium produced?
- Who discovered scandium?
- How common is scandium on Earth?
- Which other element is scandium most like?
- What are some uses of scandium today?
Answers:
- 1960
- Lars Frederik Nilson
- The 50th most common element
- Aluminum
- Bright lights, baseball bats, aircraft parts, golf clubs, and firearms